Abstract
Background: Oral anticancer (OAC) agents revolutionized the treatment of once-fatal chronic leukemias by extending survival and delaying progression; however, this requires a medication adherence rate >90%. Innovative models to improve patient knowledge and adherence to OACs exist. A critical limitation of these previous models is a lack of clear strategies to improve their adoption, implementation, and maintenance. Previously, we identified barriers and facilitators to the implementation and maintenance of a medication adherence intervention for OACs. Here, we use focus group discussions with clinical teams, administrators, and patients to design practical strategies for the program's adoption, implementation, and maintenance.
Methods: We assembled an advisory panel with clinicians (n=9), administrators (n=7), and patients (n=2) in two settings (an academic and a community cancer center) to design practical strategies for the adoption, implementation, and maintenance of a structured adherence intervention using a 5-step systematic approach called implementation mapping. We report findings from the first three steps. In step 1 (needs assessment), we conducted a consensus building discussion followed by a survey to identify perceived importance and changeability of predetermined barriers to implementation of adherence interventions (from literature review and qualitative interviews). In step 2 (determine outcomes/objectives), we identified agreeability with predetermined performance outcomes and objectives associated with the adoption, implementation, and maintenance of the program. Both surveys consisted of questions measured using a 5-point Likert scale response, ranging from 1 (not at all important/extremely difficult) to 5 (Extremely important/extremely easy). Results from these surveys were used to select and produce implementation strategies (steps 3-5) using the CFIR-ERIC Strategy Matching Tool (https://cfirguide.org/choosing-strategies/ which helps match evidence-based strategies to identified barriers) to address previously identified barriers to program implementation. Implementation strategies were further refined through focus group discussions with health care practitioners.
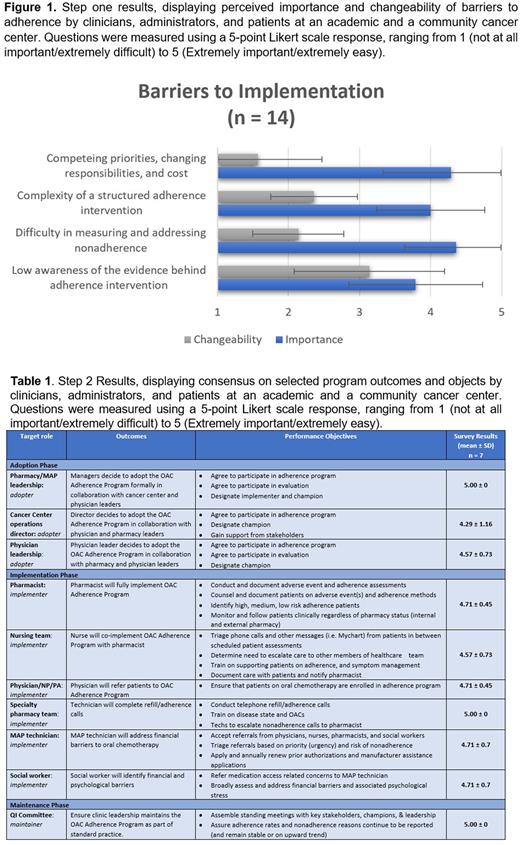
Results: Three sets of consensus-building sessions followed by surveys were conducted with the advisory panel. In the first set, after the qualitative focus group discussion, 14 participants completed a survey (Figure 1), where 4 barriers to adherence were assessed. Survey participants found all barriers surveyed to be important to address, with >70% of survey respondents selecting 4 or 5 for every barrier. However, all but one barrier was perceived as difficult to change, with low awareness of the evidence behind the adherence intervention perceived as neither easy nor difficult. In the second set, a consensus-building discussion was held with the advisory panel followed by a survey aimed at selecting program outcomes and objectives. Seven participants completed the survey (Table 1) and 10 performance outcomes and 18 performance objectives were assessed. There was moderate to high agreement (range: 4.0 to 5) on all 28 items, with >85% of participants selecting 4 or 5 on 25 of the 28 items. In the third set (focus group and survey), we aimed to identify and refine implementation strategies. Nine participants completed a survey, and 21 strategies were proposed (8 for adoption; 11 for implementation; 2 for maintenance) with moderate to high levels of agreement (range: 3.78 to 5) for all strategies. After a final focus group discussion with the advisory panel, four strategies were reached for final production: 1) Development of a standard operating procedure; 2) Creation of a short course on implementing motivational interviewing techniques to improve adherence; 3) Defining measurable performance indicators and metrics; and 4) Scheduling quarterly meetings with clinical teams.
Conclusions: Using a systematic approach and an interprofessional advisory panel, our team confirmed known and modifiable barriers to the adoption, implementation, and maintenance of a structured adherence intervention. Through quantitative surveys and qualitative focus group discussions, we built consensus program outcomes and objectives as well as practical implementation strategies. Using these results, we plan to pilot and evaluate an oral chemotherapy adherence program.
Disclosures
Collins:GlaxoSmithKline: Other: Post Doctoral Fellow sponsored by this company. Muir:GlaxoSmithKline: Current Employment. Mackler:mBIOHEALTH: Consultancy, Current equity holder in private company; AstraZeneca: Research Funding. Bryant:Carevive Systems, Inc: Research Funding. Wood:Genentech: Research Funding; Pfizer: Research Funding. Zullig:Proteus Digital Health: Research Funding; PhRMA Foundation: Research Funding; Novartis: Consultancy; Pfizer: Consultancy. Muluneh:Novartis: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Servier Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal